Metals and Nonmetals Can React With Each Other to Form Ions
9.2: Metals and Nonmetals and their Ions
- Page ID
- 24231
Learning Objectives
- To understand the basic properties separating Metals from Nonmetals and Metalloids
An element is the simplest form of matter that cannot be split into simpler substances or built from simpler substances by any ordinary chemical or physical method. There are 118 elements known to u.s., out of which 92 are naturally occurring, while the rest have been prepared artificially. Elements are farther classified into metals, non-metals, and metalloids based on their backdrop, which are correlated with their placement in the periodic table.
| Metal Elements | Nonmetallic elements |
|---|---|
| Distinguishing luster (smooth) | Non-lustrous, various colors |
| Malleable and ductile (flexible) equally solids | Brittle, hard or soft |
| Conduct heat and electricity | Poor conductors |
| Metallic oxides are basic, ionic | Nonmetallic oxides are acidic, covalent |
| Grade cations in aqueous solution | Course anions, oxyanions in aqueous solution |
With the exception of hydrogen, all elements that class positive ions by losing electrons during chemical reactions are called metals. Thus metals are electropositive elements with relatively low ionization energies. They are characterized by bright luster, hardness, ability to resonate audio and are excellent conductors of oestrus and electricity. Metals are solids under normal conditions except for Mercury.
Physical Backdrop of Metals
Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Other backdrop include:
- Country: Metals are solids at room temperature with the exception of mercury, which is liquid at room temperature (Gallium is liquid on hot days).
- Luster: Metals take the quality of reflecting light from their surface and can exist polished e.g., aureate, silver and copper.
- Malleability: Metals have the power to withstand hammering and can be made into thin sheets known as foils. For example, a sugar cube sized chunk of gold tin can be pounded into a thin sheet that volition cover a football field.
- Ductility: Metals tin can be drawn into wires. For example, 100 g of silvery can exist drawn into a thin wire about 200 meters long.
- Hardness: All metals are hard except sodium and potassium, which are soft and can be cut with a knife.
- Valency: Metals typically take ane to 3 electrons in the outermost shell of their atoms.
- Conduction: Metals are adept conductors considering they accept free electrons. Silverish and copper are the 2 best conductors of heat and electricity. Atomic number 82 is the poorest conductor of heat. Bismuth, mercury and iron are also poor conductors
- Density: Metals have high density and are very heavy. Iridium and osmium have the highest densities whereas lithium has the lowest density.
- Melting and Boiling Points: Metals have loftier melting and boiling points. Tungsten has the highest melting and boiling points whereas mercury has the lowest. Sodium and potassium also have depression melting points.
Chemical Properties of Metals
Metals are electropositive elements that generally grade basic or amphoteric oxides with oxygen. Other chemical properties include:
- Electropositive Character: Metals tend to have low ionization energies, and typically lose electrons (i.e. are oxidized) when they undergo chemical reactions They commonly do non accept electrons. For case:
- Alkali metals are always 1+ (lose the electron in s subshell)
- Alkaline earth metals are always 2+ (lose both electrons in s subshell)
- Transition metallic ions do not follow an obvious pattern, 2+ is mutual (lose both electrons in southward subshell), and i+ and 3+ are likewise observed
\[\ce{Na^0 \rightarrow Na^+ + e^{-}} \label{ane.1} \nonumber \]
\[\ce{Mg^0 \rightarrow Mg^{2+} + 2e^{-}} \label{1.two} \nonumber\]
\[\ce{Al^0 \rightarrow Al^{iii+} + 3e^{-}} \characterization{1.three} \nonumber\]
Compounds of metals with not-metals tend to be ionic in nature. Near metal oxides are bones oxides and deliquesce in water to form metal hydroxides :
\[\ce{Na2O(s) + H2O(l) \rightarrow 2NaOH(aq)}\label{1.4} \nonumber\]
\[\ce{CaO(s) + H2O(fifty) \rightarrow Ca(OH)two(aq)} \characterization{1.5} \nonumber\]
Metallic oxides exhibit their basic chemic nature past reacting with acids to form metal salts and water:
\[\ce{MgO(s) + HCl(aq) \rightarrow MgCl2(aq) + H2o(l)} \label{one.vi} \nonumber\]
\[\ce{NiO(south) + H2SO4(aq) \rightarrow NiSO4(aq) + Water(fifty)} \label{1.seven} \nonumber\]
Example \(\PageIndex{1}\)
What is the chemical formula for aluminum oxide?
Solution
Al has a 3+ charge, the oxide ion is \(O^{2-}\), thus \(Al_2O_3\).
Case \(\PageIndex{2}\)
Would you await it to be solid, liquid or gas at room temperature?
Solutions
Oxides of metals are characteristically solid at room temperature
Example \(\PageIndex{3}\)
Write the counterbalanced chemical equation for the reaction of aluminum oxide with nitric acid:
Solution
Metal oxide + acrid -> common salt + water
\[\ce{Al2O3(s) + 6HNO3(aq) \rightarrow 2Al(NO3)3(aq) + 3H2O(l)} \nonumber\]
Elements that tend to gain electrons to form anions during chemic reactions are chosen non-metals. These are electronegative elements with high ionization energies. They are non-lustrous, brittle and poor conductors of rut and electricity (except graphite). Non-metals can exist gases, liquids or solids.
Physical Properties of Nonmetals
- Physical State: Well-nigh of the non-metals exist in two of the three states of affair at room temperature: gases (oxygen) and solids (carbon). Only bromine exists equally a liquid at room temperature.
- Non-Malleable and Ductile: Non-metals are very brittle, and cannot exist rolled into wires or pounded into sheets.
- Conduction: They are poor conductors of oestrus and electricity.
- Luster: These take no metal luster and do non reflect light.
- Melting and Boiling Points: The melting points of non-metals are by and large lower than metals, merely are highly variable.
- Seven non-metals be nether standard conditions every bit diatomic molecules: \(\ce{H2(k)}\), \(\ce{N2(g)}\), \(\ce{O2(g)}\), \(\ce{F2(g)}\), \(\ce{Cl2(one thousand)}\), \(\ce{Br2(l)}\), \(\ce{I2(s)}\).
Chemic Properties of Nonmetals
Non-metals have a tendency to proceeds or share electrons with other atoms. They are electronegative in character. Nonmetals, when reacting with metals, tend to proceeds electrons (typically attaining noble gas electron configuration) and become anions:
\[\ce{3Br2(l) + 2Al(s) \rightarrow 2AlBr3(south)} \nonumber\]
Compounds equanimous entirely of nonmetals are covalent substances. They by and large form acidic or neutral oxides with oxygen that that dissolve in water to grade acids:
\[\ce{CO2(g) + H2O(l)} \rightarrow \underset{\text{carbonic acrid}}{\ce{H2CO3(aq)}} \nonumber\]
As you may know, carbonated h2o is slightly acidic (carbonic acid).
Nonmetal oxides can combine with bases to form salts.
\[\ce{CO2(m) + 2NaOH(aq) \rightarrow Na2CO3(aq) + H2O(l)} \nonumber\]
Metalloids have properties intermediate between the metals and nonmetals. Metalloids are useful in the semiconductor manufacture. Metalloids are all solid at room temperature. They tin form alloys with other metals. Some metalloids, such every bit silicon and germanium, can act as electric conductors under the right conditions, thus they are chosen semiconductors. Silicon for example appears lustrous, but is not malleable nor ductile (information technology is brittle - a characteristic of some nonmetals). It is a much poorer conductor of heat and electricity than the metals. The concrete properties of metalloids tend to exist metal, but their chemical properties tend to be non-metallic. The oxidation number of an element in this group tin can range from +5 to -2, depending on the grouping in which it is located.
| Metals | Not-metals | Metalloids |
|---|---|---|
| Gilded | Oxygen | Silicon |
| Silver | Carbon | Boron |
| Copper | Hydrogen | Arsenic |
| Iron | Nitrogen | Antimony |
| Mercury | Sulfur | Germanium |
| Zinc | Phosphorus |
Metallic character is strongest for the elements in the leftmost office of the periodic table, and tends to decrease as we move to the right in whatsoever catamenia (nonmetallic grapheme increases with increasing electronegativity and ionization energy values). Within any grouping of elements (columns), the metal character increases from top to bottom (the electronegativity and ionization free energy values generally decrease as we move down a grouping). This general trend is not necessarily observed with the transition metals.
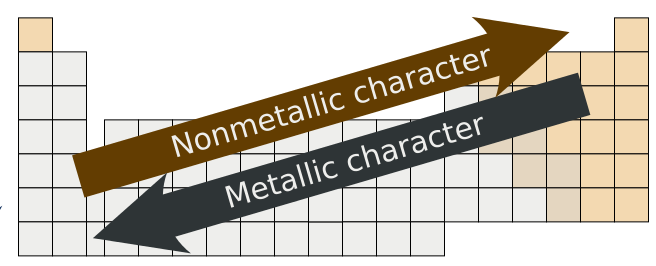
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/09%3A_The_Periodic_Table_and_Some_Atomic_Properties/9.2%3A_Metals_and_Nonmetals_and_their_Ions
Post a Comment for "Metals and Nonmetals Can React With Each Other to Form Ions"